

Entia non sunt multiplicanda praeter necessitate.
William of Ockham (c. 1285-1349), author of the Ockham’s Razor principle.
PRE-CLINICAL RESEARCH AND DEVELOPMENT
Our focus
We are focussed on the use of heparin and its derivatives for respiratory diseases, the epidemiology and commercial prospects for which are described in detail on our page for Investors.
Background
Pre-clinical research has established the mucolytic activity of heparin. Patients with obstructive inflammatory airways diseases, such as cystic fibrosis (CF), bronchiectasis and COPD often have abnormally high levels of extracellular DNA in the airway secretions (Figure 1A), that form a polymeric network contributing to the viscosity of the secretions making them difficult to expectorate. Retained secretions limit the delivery of inhaled drugs that target the airway epithelium and underlying tissues and invite infection that further promote the inflammatory response. DNA also inhibits the activity of a number of antibiotics and supports the growth of bacterial biofilm. The removal of extracellular DNA therefore is a clinical imperative.
Ockham Biotech's research has shown that, when treated with heparin (Figure 1B) DNA networks dissolve and the thinned mucus is more easily expectorated. This is in part due to the activation of endogenous DNase I. Our research has also shown that heparin activates DNase I added exogenously to DNA networks.
Inhaled heparin leads to the opportunity to improve mucus clearance and the delivery of other inhaled drugs, including corticosteroids and bronchodilators, to underlying cells and tissues.
In addition to its mucolytic properties, heparin possesses a multitude of other effects broadly grouped into antimicrobial and antiinflammatory properties.
In 2016, a Phase IIb trial investigated the influence of inhaled heparin over 21 days on pulmonary function and exercise capacity amongst 24 moderate to severe COPD patients. The results of this study showed significant increases in lung function as measured by forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), and significantly reduced air trapping as measured by inspiratory capacity (IC), total lung capacity (TLC) and residual volume (RV) against placebo (Figure 1C). These results were maintained for at least 7 days after treatment was stopped. Importantly, exercise parameters showed a clinically significant improvement in the distance covered during a 6 minute walking test and a significant reduction in post-exercise dyspnoea. A review published in 2023 brought together this trial with others showing the benefits of inhaled heparin in COPD.
In addition, there is clinical evidence of the benefit of nebulised unfractionated heparin (UFH) in infectious lung conditions such as COVID-19. In early-phase trials in patients with acute lung injury, nebulised UFH reduced microvascular thrombosis and hypercoagulation, improved pulmonary dead space and reduced lung injury, with increased time free of ventilatory support. In a further exploratory trial in Brazil, nebulised UFH on top of standard of care for patients hospitalised with COVID-19 showed clinical benefit. The primary endpoints showed a reduction in mortality, reaching significance in the mITT population, but no difference in duration of hospital stay.
Despite the clear evidence supporting the benefit of inhaled heparin in various lung conditions and its demonstrable safety, the perceived risks of administration of an anticoagulant directly into the lungs of a patient remained a barrier to widespread adoption of this therapy.
OCK4
OCK4 is a heparin analogue that lacks the anticoagulant effects of heparin, while still retaining the mucolytic, antimicrobial and anti-inflammatory properties. It is therefore a highly attractive drug for use in obstructive airways diseases.
OCK4 has previously been administered to humans for another (non-respiratory) purpose, which has established its excellent safety. We are now in the process of undertaking some additional pre-clinical toxicology to establish its safety by the inhaled route of administration, which should enable the start of our clinical trials.
OCK4 has multiple pharmacological properties and is proposed to inhibit mucus secretion and improve mucus clearance through its anti-inflammatory effects as well as direct effects on mucus rheology. Antimicrobial effects are proposed to be through mechanisms that avoid bacterial resistance.
In vitro experiments confirmed that OCK4 inhibited human neutrophil elastase activity and activated DNase (Data on File).
IIn a rat model of chronic Pseudomonas aeruginosa-induced pneumonia, OCK4 reduced the adverse outcome on mean body weight and body temperature, while significantly reducing cytokine concentrations and neutrophil elastase activity in bronchoalveolar lagage fluid (Data on File).
Nebuliser
Ockham Biotech is working with a mesh nebuliser manufacturing company to develop a specific device capable of delivering therapeutic quantities of OCK4 via a patient hand-held device in less than 10 minutes.
In addition, the aerosol generated by this device is being analysed for its aerodynamic properties and its predicted distribution in both NCFBE and CF patients.
Figure 1;
A
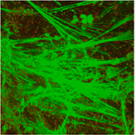
B

C
